You are about to leave this website for another external website outside of the control of Gamida Cell. Gamida Cell has no responsibility for the content of such other sites and is not liable for any damages or injury arising from that content. Any links to other sites are provided merely as a convenience to the users of this website.
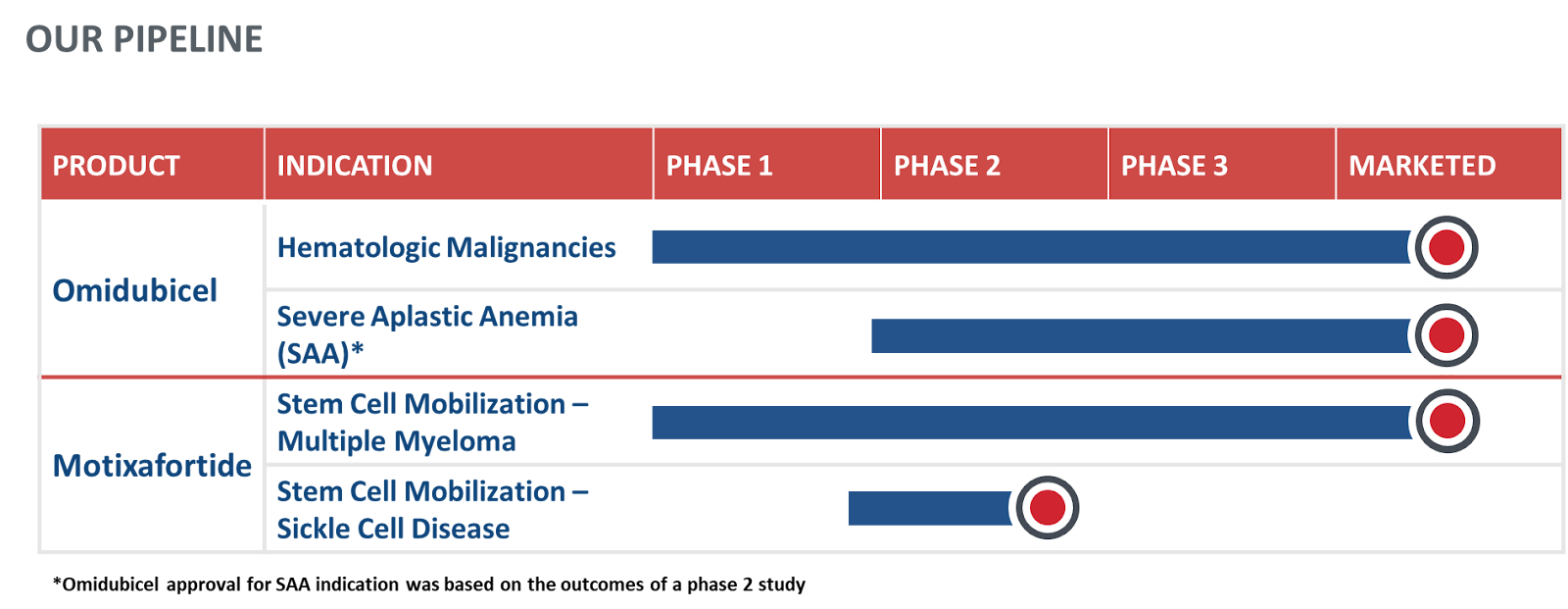
PIPELINE
Driving innovative therapeutics across transplantation
Our proprietary nicotinamide (NAM) technology enhances and expands innate stem cells, offering potentially curative options for patients living with blood cancers and severe aplastic anemia (SAA).
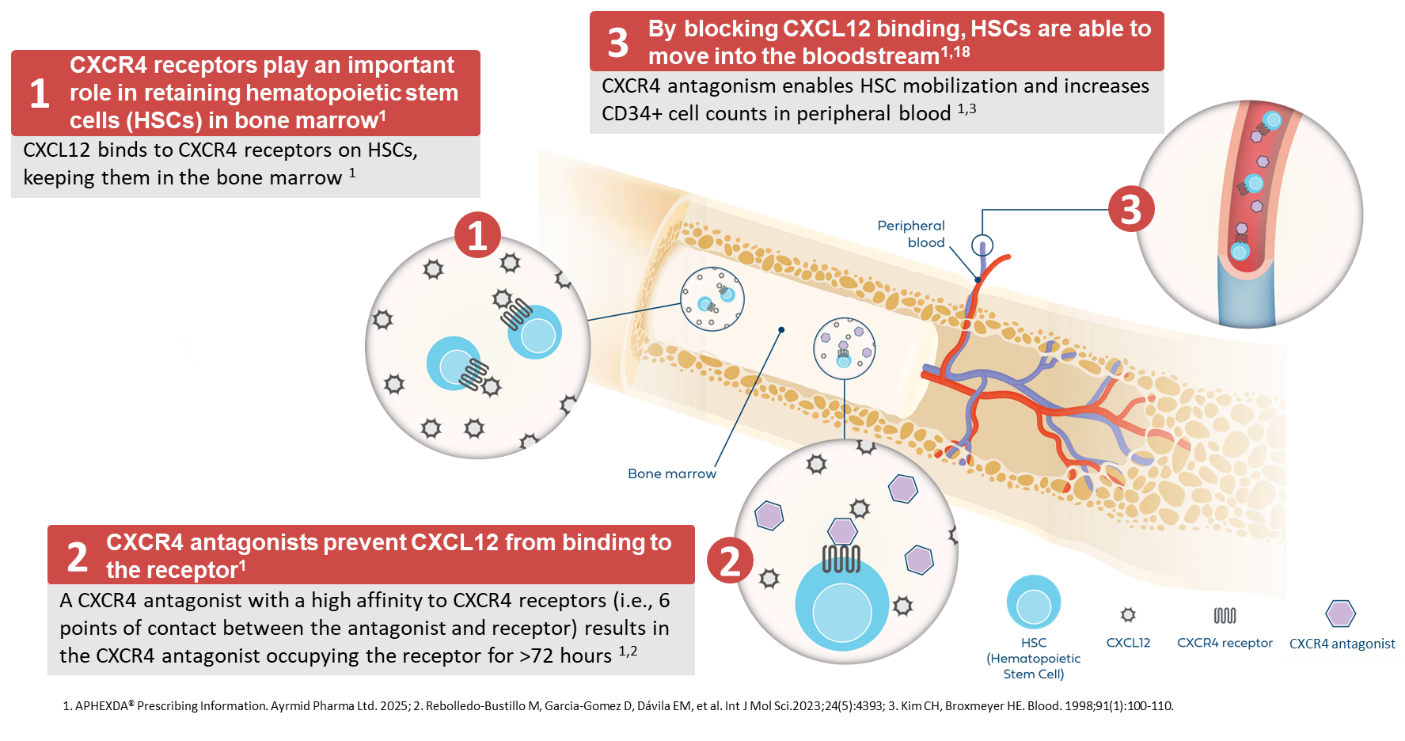
CXCR4 antagonist mobilizes stem cells in patients with Multiple Myeloma who then receive these cells to treat their condition through transplantation. Our CXCR4 antagonist is also being studied to support stem cell mobilization for patients with sickle cell disease who are designated for treatment with gene therapy.
Explore our pipeline below for information on our clinical development candidates and ongoing clinical trials.
The safety and efficacy of motixafortide for stem cell mobilization in Sickle Cell Disease has not been established by the U.S. Food and Drug Administration or any other health authority.
Motixafortide
Motixafortide, already FDA approved for stem cell mobilization in multiple myeloma, is being evaluated in ongoing investigator initiated studies (NCT06442761 and NCT05618301) evaluating CD34+ Hematopoietic Stem Cells for Gene Therapies in Sickle Cell Disease (SCD).
For Full Prescribing Information on APHEXDA in Multiple Myeloma, please visit aphexda.com.
The safety and efficacy of motixafortide in sickle cell disease has not been established by the U.S. Food and Drug Administration or any other health authority.
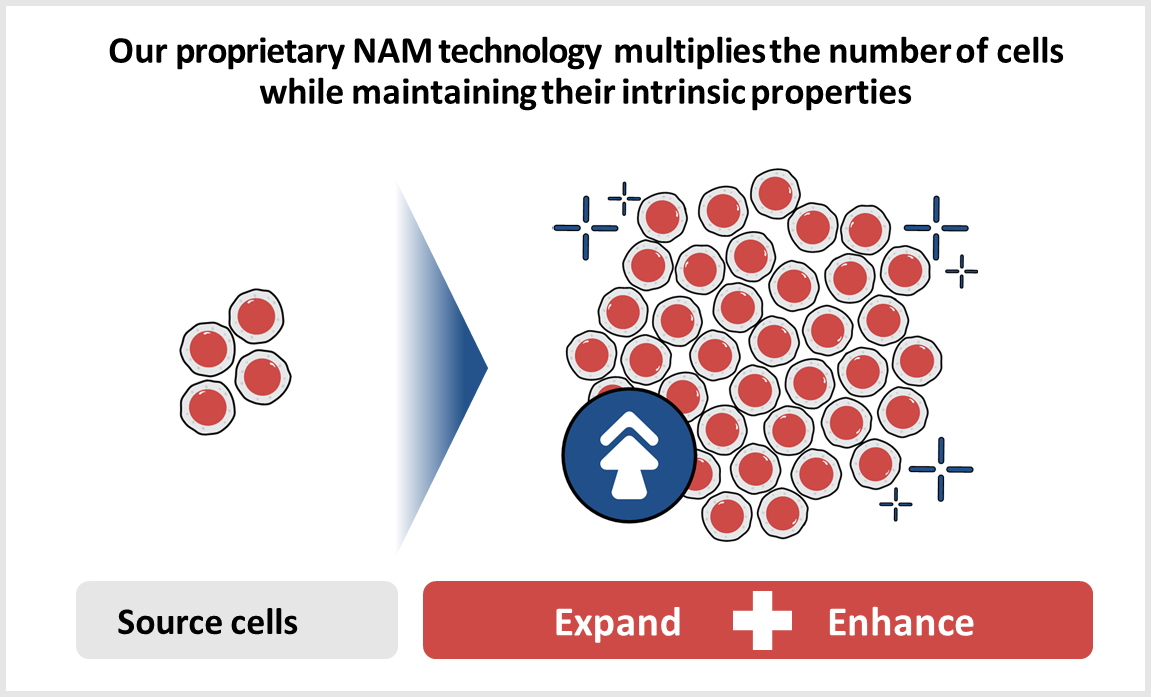
NAM Technology
Gamida Cell’s proprietary nicotinamide (NAM) technology expands the number of stem cells while maintaining their intrinsic properties and enhances cellular functionality and phenotype. For stem cells, this may mean improved homing and retention in bone marrow and lymph nodes and increased metabolic fitness. Our technology leverages the properties of NAM, creating allogeneic cell therapy products and candidates that are potentially curative for patients with hematologic malignancies and in Severe Aplastic Anemia.
CXCR4 Antagonist Mechanism of Action
OUR MANUFACTURING
Gamida Cell has a wholly owned, fully licensed GMP manufacturing facility in Israel and have strategic partnership in place for future manufacturing of OMISIRGE® at RoslinCT in Hopkinton, MA.
Selected Publications and Presentations
2025
Blood 2025; 146 (Supplement 1): 2542.
A first-in-human, safety and feasibility study. Blood 2025; 146 (Supplement 1): 5956.
Blood 2025; 146 (Supplement 1): 5957.
Blood 2025; 146 (Supplement 1): 5963.
Blood Neoplasia. 2025 2(3):100104.
Transplant Cell Ther. 2025 Jul;31(7):436-447.
2024
2023
Transplant Cell Ther. 2023 Dec;29(12):749.e1-749.e5.
Transplant Cell Ther. 2023 Aug;29(8):517.e1-517.e12.
Transplant Cell Ther. 2023 May;29(5):338.e1-338.e6.
Transplant Cell Ther. 2023 Jan;29(1):52.e1-52.e9.
2022
Health-Related Quality of Life Following Allogeneic Hematopoietic Stem Cell Transplantation with Omidubicel Versus Standard Umbilical Cord Blood
Presented at the Tenth Annual Meeting of the Society of Hematologic Oncology
2021
Blood. 2021 Oct 21;138(16):1429-1440.

